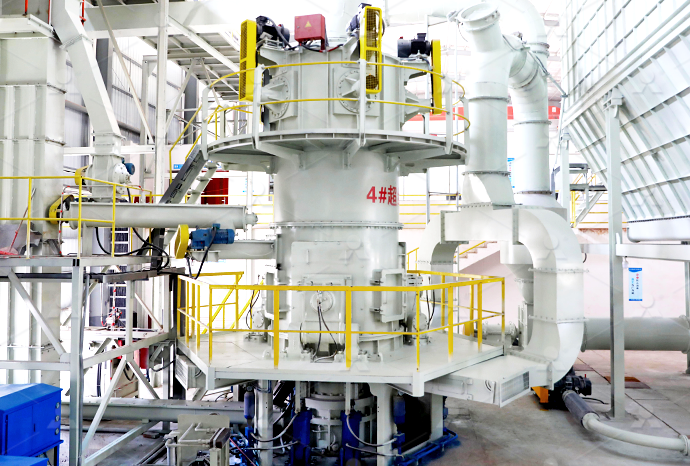
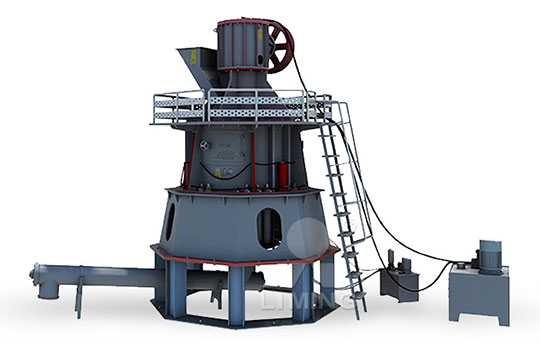
Conversion of limestone ore raw stone to calcium carbonate volume

limestone, quicklime and slaked lime chemguide
Limestone, quicklime and slaked lime This page looks at the origin and uses of limestone, and its conversion into quicklime, CaO, and slaked lime, Ca(OH) 2 Limestone and marble Chemically, limestone is calcium carbonate It is a sedimentary rock formed from the shells and skeletons Using chips of limestone rocks, students prepare a powdered sample of limestone, react it with an excess of HCl, and determine calcium carbonate content of the limestone by backtitration of EXPERIMENT Calcium Carbonate Content of 01 Chem21LabsCarbonation experiments of calcined natural limestone particles were carried out in a thermal gravimetric analyzer and a tube furnace to investigate the effects of carbonation temperature, Experimental study and modeling of CaO carbonation: Effects of 2021年10月4日 During the flue gas purification, lime reacts with HCl, SOx and HF but also with CO 2, forming calcium carbonate A carbonation rate in APCR of about 30% is reported, which Natural and enhanced carbonation of lime in its different
.jpg)
Multicyclic conversion of limestone at Calooping conditions:
Limestone derived CaO conversion when subjected to multiple carbonation/calcination cycles is a subject of interest currently fueled by several industrial applications of the socalled Calooping Conversion of Recommended Calcium Carbonate Equivalent to Recommended Limestone CCE (lb/A) Recommended by Soil Test Percent Calcium Carbonate Equivalent (% CCE) of Liming Table B3 Conversion of Recommended Calcium Carbonate 2005年11月1日 The grain structure of the parent stone remained after calcination, and the domains were recognisable even after 40 calcination/carbonation cycles The key parameter Review—calcination and carbonation of limestone during thermal 2014年7月1日 Limestone derived CaO conversion when subjected to multiple carbonation/calcination cycles is a subject of interest currently fueled by several industrial Multicyclic conversion of limestone at Calooping conditions: The
Sintering of Limestone in Calcination/Carbonation Cycles
The sintering of CaO is accelerated by higher temperatures and that will lead to the lower carbonation conversion ratios for the CaO The highest carbonation ratio is not obtained for 2015年7月21日 The calcination reaction of limestone (CaCO 3) to yield lime (CaO) is at the heart of many industrial applications as well as natural processes In the recently emerged Crystallographic transformation of limestone during calcination At 1200K, calcium carbonate decomposes to give carbon dioxide and calcium oxide CaCO 3 → CaO + CO 2; On reacting with dilute acids, calcium carbonate gives carbon dioxide CaCO 3 + 2HC l → CaCl 2 + H 2 O +CO 2; Application Limestone: Calcium Carbonate (CaCO3) Uses, Limestone is part of the charge used when extracting iron from its ore Limestone is primarily composed of a calcium carbonate compound and is not referred to as coke When calcium carbonate decomposes as a result of the high heat inside the furnace, it reacts with sandy impurities to form what is known as slagLesson Explainer: Extracting Iron Nagwa

PRECIPITATED CALCIUM CARBONATE PRODUCTION, SYNTHESIS
Precipitated calcium carbonate production, synthesis and properties 59 al (2008) and Lim et al (2010) have investigated mineral carbonation with carbon dioxide gas Teir et al (2007) investigated dissolution properties of steelmaking slags in acetic acid for precipitated calcium carbonate production Effect of limestone2016年1月1日 Using phosphogypsum (PG) as a raw material for the production of valuable products is interesting In this work we present the wet chemical conversion of phosphogypsum to calcium carbonate and Conversion of phosphogypsum to sodium sulfate and calcium carbonate Acta Chimica Slovaca, 2019 Reactivity of various calcium carbonate samples for flue gas desulfurization was tested Two groups of CaCO3 samples were considered; natural limestone containing calcite phase dominantly and samples prepared by the conversion of gypsum with ammonium and carbon dioxide (precipitated CaCO3) containing different amounts of calcite, Conversion of calcium sulphide to calcium carbonate during the process 2014年8月13日 The production of elemental sulphur and calcium carbonate (CaCO3) from gypsum waste can be achieved by thermally reducing the waste into calcium sulphide (CaS), which is then subjected to a direct Conversion of calcium sulphide to calcium carbonate during the process
.jpg)
Limestone Types, Properties, Composition, Formation, Uses
2023年10月21日 Limestone is a sedimentary rock primarily composed of calcium carbonate (CaCO3) in the form of mineral calcite or aragoniteIt is one of the most common and widely distributed rocks on Earth, with a wide range of uses in various industries and natural settings Limestone forms through the accumulation and compaction of marine organisms, primarily the 2008年9月17日 Request PDF Steel Converter Slag as a Raw Material for Precipitation of Pure Calcium Carbonate Industrial Engineering Chemistry Research Vol47, 71047111 In this work we study the Steel Converter Slag as a Raw Material for Precipitation of Pure 2017年1月1日 PDF Calcium carbonate (CaCO3) is the most widely used filler material in paper, paint, plastic, food, ceramic, cosmetic, medicine and other Find, read and cite all the research you need on Precipitated Calcium carbonate production, synthesis and propertiesCalcium Carbonate lumps Limestone raw lumps CaCo3 98 % + Lab Reports Attached in Image Please Check GET QUOTESCalcium Carbonate Limestone Raw QUAZI MINES MINERALS

Calcium Oxalate: A Surface Treatment for Limestone
1998年5月1日 Calcium Oxalate: A Surface Treatment for Limestone Tody M Cezar Architectural Stone Carving Course The City and Guilds of London Art School, 124 Kennington Park Road, London, SE11 4DJ, United Kingdom 2012年2月1日 A pesar de esto, se reportan pocas investigaciones en la literatura especializada sobre la evaluación y/o optimización del consumo de energía en este proceso [3,14,16,17,18]The Kinetics of Calcination of High Calcium The practice of burning limestone to produce quicklime is, almost literally, as old as the hills In terms of basic chemistry and materials, the process involves the conversion of calcium carbonate, CaCO 3, to the more useful calcium oxide, CaO Calcium oxide is a very reactive substanceHOW TO CALCULATE EFFICIENCY OF YOUR LIME BURNING 1 cubic meter of Calcium Carbonate weighs 2 711 kilograms [kg] 1 cubic foot of Calcium Carbonate weighs 1692422 pounds [lbs] Calcium Carbonate weighs 2711 gram per cubic centimeter or 2 711 kilogram per cubic meter, ie density of calcium Carbonate is equal to 2 711 kg/m³; at 252°C (7736°F or 29835K) at standard atmospheric pressureCalcium Carbonate volume to weight conversion
.jpg)
The wet conversion of phosphogypsum by ammonium carbonate
2023年3月1日 The PG conversion by ammonium carbonate ((NH 4) 2 CO 3) into calcium carbonate (CaCO 3 ) and ammonium sulfate ((NH 4 ) 2 SO 4 ) is classifying in the top of the PG conversion and it’sCrystal structure of calcite Calcium carbonate shares the typical properties of other carbonatesNotably it reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water:; CaCO 3 (s) + 2 H + (aq) → Ca 2+ (aq) + CO 2 (g) + H 2 O(l) releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above Calcium carbonate Wikiwand1 Introduction Biomineralization processes demonstrate that it is possible to achieve exceptional control over crystallization This is wellillustrated by the calcium carbonate system, in which organisms select the polymorph with perfect fidelity, tune the structure from single crystals to polycrystalline materials, generate complex morphologies and control orientationMagnesium Ions Direct the Solid‐State Transformation of About Limestone; 2 711 kilograms [kg] of Limestone fit into 1 cubic meter; 1692422 pounds [lbs] of Limestone fit into 1 cubic foot; Limestone weighs 2711 gram per cubic centimeter or 2 711 kilogram per cubic meter, ie density of limestone is equal to 2 711 kg/m³; at 252°C (7736°F or 29835K) at standard atmospheric pressureLimestone weight to volume conversion AquaCalc

Preparation of Precipitated Calcium Carbonate from Wadi Ghadaf
Iraqi Bulletin of Geology and Mining Vol11, No2, 2015 p 123 138 PREPARATION OF PRECIPITATED CALCIUM CARBONATE FROM WADI GHADAF LIMESTONE OF DAMMAM FORMATION Wasan A Muslim1 and Alaa M Kh Mustafa2 Received: 04/ 05/ 2014, Accepted: 02/ 12/ 2014 Key words: Precipitated calcium carbonate, Limestone, Calcination, Slaking, as one of the raw materials which go into the blast furnace in the extraction of iron from its ore In neutralising acids: Limestone has been used to treat lakes acidified because of acid rain; There is a useful bit of video which shows the conversion of calcium carbonate into calcium oxide and then calcium hydroxide It is actually quite limestone, quicklime and slaked lime chemguidegeological formation Limestone is generally classified into the following types: a) High calcium: The carbonate content is composed essentially of calcium carbonate with a magnesium carbonate content of no more than 5%(usually less) b) Magnesian: This contains magnesium carbonate to The Kinetics of Calcination of High Calcium Limestone IDCOnline2021年7月22日 The present study was conducted to evaluate the effects of limestone replacement with dietary microcalcium carbonate (MCC) on performance, egg quality, and serum Ca metabolism in peakphase (PDF) Effects of partial replacement of conventional
.jpg)
limestone, quicklime and slaked lime
2023年10月11日 At Mississippi Lime, our calcium oxide (CaO), or commonly referred to as quicklime, is a high calcium content derivative of our 985% pure calcium carbonate (limestone) Calcium oxide is produced by heating calcium carbonate to 2000 degrees F where carbon dioxide disassociates as a gas from the calcium carbonate:The paper presents application of calcium carbonate, which is included in the limestone, for wet limestone method of desulphurization used for flue gas generated in hard and brown coal combustion in pulverized coal furnaces of power boilers Conversion stages of calcium carbonate into calcium sulphate dihydrate, ie synthetic gypsum, are givenConversion of calcium carbonate into synthetic gypsum in flue 2012年4月23日 The conversion of phosphogypsum waste (a waste product of phosphoric acid production in Richard Bay, South Africa), using sodium carbonate was tested Response surface methodology (RSM) was used to investigate the combined effect of relevant process variables to maximize the production of calcium carbonate in a batch reactor The process variables Phosphogypsum Conversion to Calcium Carbonate and 2024年5月24日 The new technology of microbially induced calcium carbonate precipitation (MICP) has been applied in construction materials as a strategy to enhance their properties In pursuit of solutions that are more localized and tailored to the study’s target, this work focused on isolating and selecting bacteria capable of producing CaCO3 for posterior application in Comparison of calcium carbonate production by bacterial
.jpg)
TEIR, S, ELONEVA, S, ZEVENHOVEN, R, 2005 Production of TKK
ufacture precipitated calcium carbonate (PCC), which is synthetic calcium carbonate (CaCO 3) with a higher purity than naturally occurring calcium carbonate (limestone) In the PCC process, purchased calcium oxide (CaO, lime) is hydrated into calcium hydroxide (Ca(OH) 2, slaked lime), followed by carbonation of the hydroxide2018年11月12日 The TG/DTA curves of the limestone samples, showing a close similarity to that of pure calcium carbonate, exhibited an endothermic peak between 600 and 760°C, with the maximum near 750°C(PDF) Characterization of quicklime as raw material to hydrated 2015年7月1日 The effect of limestone filler (up to 20%) on the degree of hydration, the volume of hydration products, and the optimal replacement of limestone filler in cement pastes at different w/cm ratios (PDF) Effect of Calcium Carbonate Replacement on Workability and 2022年1月18日 The production of precipitated calcium carbonate (PCC) from carbonatefree minerals and CO2 from industrial exhaust gas could lower energy consumption and promote CO2 sequestration The present study proposes a chemical looping process for the production of PCC from wollastonite (calcium silicate) and CO2 from industrial exhaust using ammonium chloride Preparation of precipitated calcium carbonate using wollastonite
.jpg)
(PDF) NearInfrared Spectroscopy of Limestone Ore for
2017年10月13日 Correlation between the absorption wavelength of 2350 nm (4255 cm −1 ) calcite absorption feature and CaO content when the rock surface is (a) dry and (b) wet2015年6月5日 As shown in Figure 6, under the same basicity, the internal structure of the limestone vanadium−titanium pellets was porous and the CO2 released by the thermal decomposition of calcium carbonate Effect of Adding Limestone on the Metallurgical Properties of Iron Ore 2019年5月1日 Thus, the normal temperature range providing the clay minerals dehydroxylation at 600800 °C is insufficient for the complete decarbonization of C/M carbonates However, calcium carbonate began Thermal decomposition of calcium carbonate (calcite Description: Carmeuse is a leading producer of ground calcium carbonate Calcium carbonate (CaCO 3) is a very common chemical compound making up 4% of the earth’s crust and is the primary constituent in limestoneWhile it is abundant, not all limestone is suitable for ground calcium carbonate (GCC) production due to impuritiesGround Calcium Carbonate Carmeuse

The Availability of Limestone and Other Raw Materials for Ocean
2022年5月25日 classification based on the carbonate content (Oates, 2002): limestone is divided in ultrahigh calcium limestone (>975% CaCO 3 ) and highcalcium limestone (95%–975% CaCO 3 ); highpurity 2007年4月1日 The same hydrate phases for both the CEM I 525 R and CEM II/ALL 525 R paste with a w/cratio of 055 were predicted by the thermodynamic model (see Figure 2 Figure 3)The Role of Calcium Carbonate in Cement Hydration2012年3月5日 Most notably, calcium carbonate (CaCO 3 , an alkaline earth carbonate) helps to regulate the carbon dioxide balance in the Earth's atmosphere [1, 2] through cycles of carbonate dissolution and Biomineralizationbased conversion of carbon dioxide to calcium In its natural form calcium carbonate occurs as limestone, a rock containing at least 50 % calcium carbonate In 2018, a pproximately 75 % of domestic crushed stone produced was limestone (USGS, 202 2 ) Limestone deposits are found throughout the world , and are recovered by quarrying or mining Generally,Calcium Carbonate Limestone US Environmental Protection
.jpg)
Conversion of calcium carbonate into synthetic gypsum in flue
The paper presents application of calcium carbonate, which is included in the limestone, for wet limestone method of desulphurization used for flue gas generated in hard and brown coal combustion in pulverized coal furnaces of power boilers Conversion stages of calcium carbonate into calcium sulphate dihydrate, ie synthetic gypsum, are givenCalcium carbonate limestone, which is also recognized as the chemical compound CaCO3, makes up almost five percent of the earth’s crust and is found all throughout the planet Calcium carbonate’s most common natural forms are limestone and marbleWhat is Calcium Carbonate Limestone? Carmeuse