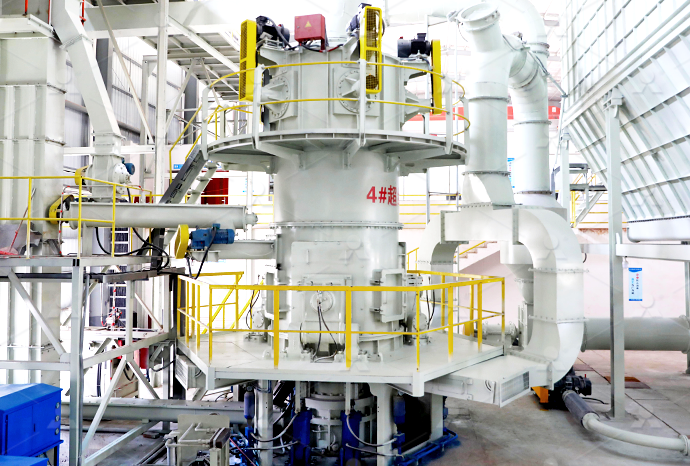
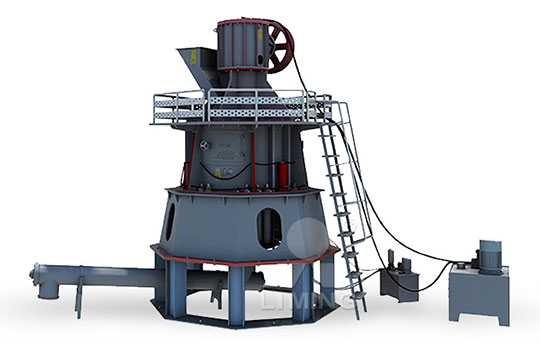
Quick lime calcium oxide kaolin powder making process
.jpg)
How to Make Quicklime: 10 Steps (with Pictures)
2024年4月16日 Here you will begin the process of turning your rock mixture into quicklime Heat your calcium carbonate directly on the flame until it becomes 2023年10月11日 Quicklime, also referred to as lime (calcium oxide (CaO)), is derived from high quality, natural deposits of limestone (calcium carbonate (CaCO3)) or dolomitic limestone Quick Lime Preparation, Properties and Uses Hebei Yayang 2024年9月21日 Production Process Calcium oxide can produced from calcium carbonate by calcination In calcination, calcium carbonate from limestone, coral, sea shells, or chalk is Calcium Oxide Quicklime Chemical Formula, UsesThe production of high calcium quicklime begins with the selective mining of chemically suitable calcium carbonate Throughout the manufacturing process, we carefully monitor the process to Quicklime Graymont

An Overview of Lime Slaking and Factors That Affect the Process
To manufacture calcium hydroxide the limestone calcium carbonate must be converted to calcium oxide and the calcium oxide then converted to calcium hydroxide The following is a brief 2023年2月4日 How lime is produced – making lime from limestone Lime is produced by decomposing calcium carbonate at temperatures around 9001100 Celsius Calcium carbonate is calcined to produce lime and generate carbon Lime/quicklime for metallurgy – how producing and Processing and grinding the quicklime produces a fine powder for multiple applications Another method for synthesizing quicklime involves heating Calcium Hydroxide, also known as slaked Quicklime (Calcium Oxide) CaO, KemicalinfoThe production of high calcium quicklime begins with the selective mining of chemically suitable calcium carbonate typically containing less than 5% magnesium carbonate Throughout the manufacturing process, we carefully High Calcium Quicklime Graymont
.jpg)
Quicklime Formula, Uses, Definition Britannica
2024年11月8日 Quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxide At room 2024年9月26日 Learn more about Calcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked lime) in detail with notes, formulas, properties, uses of Calcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked lime) prepared by subject matter experts Download a free PDF for Calcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked lime) to clear your doubtsCalcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked limePerfect for making lime putty hotmixed lime mortars Available as a fine powder or as pebbles from 515mm a chemical change in a kiln, liberating it of all the carbon and water it holds, creating a very unstable material (calcium Buy Quicklime Online Powder Pebble Cornish LimeQuick lime is used in the manufacture of iron and steel, and paper and pulp A combination of phenolphthalein and calcium oxide is used in water detection pastes Used in making porcelain and glass In preparation of bleaching powder, calcium carbide, and calcium cyanamide In water softeners Also in mortars and cementQuicklime Properties, Uses and Application Vedantu
.jpg)
Quick lime lump, Burnt lime,Calcium cacbonate ,Calcium oxide ,Quick
Tan Phuong Nam Co, Ltd is one of the leading manufacturers in Vietnam lime in mining, processing and export of minerals such as Quick lime lump, Burnt lime, Calcium cacbonate ,Calcium oxide , Quick lime , Calcium cacbonate Powder ,Calcium oxide Powder, Hydrated lime is used widely in paper industry, steel, chemicals, water treatment In addition, our company Quicklime is an alternate name for the chemical compound known as calcium oxide This compound is represented by the chemical formula CaO and is also known as burnt lime quicklime exists as a white to pale yellow powder Take up a quiz on Quick lime Q 5 Put your understanding of this concept to test by answering a few MCQsQuicklime Preparation, Properties, and Applications with FAQs2023年11月9日 Burning (calcination) of calcium carbonate in a lime kiln above 900 °C (1,650 °F)[4] converts it into the highly caustic material burnt lime, unslaked lime or quicklime (calcium oxide) and, through subsequent addition of water, into the less caustic (but still strongly alkaline) slaked lime or hydrated lime (calcium hydroxide, Ca(OH)2), the process of which is called Differences Between Hydrated Lime and QuicklimeCalcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO 3; mineral calcite) in a lime kilnThis is accomplished by heating the material to above 825 °C (1,517 °F), [6] [7] a process called calcination or limeburning, to liberate a molecule of carbon dioxide (CO 2), leaving quicklime Calcium oxide Wikiwand

Calcium Oxide Properties, Uses and Preparation of Calcium Oxide
Calcium oxide is manufactured by heating coal, limestone, seashells, or chalk These materials are highly concentrated which drives off carbon dioxide when heated This results in the production of calcium oxide The process of obtaining calcium oxide is reversible when it reacts with carbon dioxide to form calcium carbonateCalcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compoundIt is a white, caustic, alkaline, crystalline solid at room temperatureThe broadly used term lime connotes calciumcontaining inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominateCalcium oxide WikipediaMicrolime is a fine, white calcium oxide powder that is made by pulverising and air classifying quicklime It is typically used in flue gas systems, for aerated concrete block manufacture, traditional mortars and animal feed Conforms to EN12518 Drinking Water Standard Quicklime – Singleton Birch2021年5月20日 Calcium oxide (and calcium hydroxide) is also an important chemical for raising the pH of potable water and wastewater during its treatment However, there are different methods used to utilize quicklime during the Calcium Oxide: From Ancient Warfare to Modern
.jpg)
QUICKLIME CALCIUM OXIDE POWDER
This reaction is called slaking of lime; It has a very high alkaline pH of around 125, making it strongly caustic and able to damage skin, eyes, and respiratory tract on contact Proper precautions must be taken when handling it; Calcium oxide (CaO) content – The highest quality burnt lime will be at least 90% pure calcium oxideCalcium Oxide + Water → Calcium Hydroxide (slaked lime) + Heat CaO + H 2 O → Ca(OH) 2 + Heat If you are wondering how to make quicklime at home, then all you need is something made of calcium carbonate, such as a piece of chalk or some seashells Heat this over a flame until it turns red hot, and there, you have just made yourself some What is Quicklime and How is it Made? Science StruckQuicklime, CaO, and slaked lime, Ca(OH) 2 When calcium carbonate is heated strongly, it decomposes to give calcium oxide and carbon dioxide CaCO 3 (s) CaO(s) + CO 2 (g) Calcium oxide is traditionally known as quicklime If you add water to calcium oxide, you get calcium hydroxide (slaked lime) CaO(s) + H 2 O(l) Ca(OH) 2 (s)limestone, quicklime and slaked lime chemguideprocess of adding water to calcium oxide to produce calcium hydroxide is referred to as hydration process or lime slaking The hydration of CaO, commercially referred to as quick lime, is an exothermic process releasing a great quantity of heat This hydration process when done with just the right amount of water is called “Dry Hydration”An Overview of Lime Slaking and Factors That Affect the Process
.jpg)
The Amazing Uses of Quicklime You Probably Didn’t Know
Property : Calcium oxide reacts with carbon dioxide to generate calcium carbonate Use: Making Mortar ☞ Calcium oxide is an essential compound to manufacture cement and mortar It is mixed with water and sand to form a stiff paste called mortar This mortar is used for cementing bricks/stones while constructing buildings2024年11月8日 The most common form of glass, sodalime glass, is composed of about 70 percent silica (silicon dioxide), 15 percent soda (sodium oxide), and 9 percent quicklime, with much smaller amounts of various other compoundsThe soda serves as a flux to lower the temperature at which the silica melts, and the quicklime acts as a stabilizer for the silicaQuicklime Formula, Uses, Definition BritannicaHigh Calcium Quicklime Pebble and Powder, used for making lime putty and hotmixed or hotlime Mortars Quicklime is Limestone (a rock rich in calcium carbonate) that has undergone a chemical change in a kiln, liberating it of all the carbon and water it holds, creating a very unstable material (calcium oxide) which needs to hydrate Quicklime will do so very energetically with High Calcium Quicklime Pebble and Powder by Cornish Lime2023年2月2日 Quick Lime (CaO) or calcium oxide, is a white or gray powder material that is applied for industries such as industrial and environmental applicationsQuicklime has various grades that are applied for differing applications In this article, we are going to take a further look at what is quicklime, its properties, its applications or uses, its types or grades its importance What Is Quick Lime? Types, Properties, And Uses Chemtradeasia
.jpg)
Differences between Hydrated lime and quicklime Sodimate Inc
2012年1月27日 The main differences between hydrated lime and quicklime are their reactivity their chemical composition Hydrated lime and quicklime are both calcium compounds In its hydrated state, calcium is called calcium hydroxide, and in its pure state it is called calcium oxide, or quicklime Calcium oxide has a heavy density (65lb/ft³) and is more reactive than hydrated 2024年7月30日 Preparation of Calcium Oxide Calcium oxide is prepared by the process called calcination Calcination involves heating calcium carbonate (CaCO 3) to a high temperature, causing it to decompose and release carbon dioxide (CO 2), leaving behind calcium oxide (CaO) as the final product CaCO 3 (g) → CaO(s) + CO(g)Calcium Oxide Formula, Properties, Preparation and UsesThroughout the manufacturing process, we carefully monitor the process to produce a high calcium quicklime that is highly reactive and has suitable particle surface area The result is a high calcium lime that will provide reliable performance for acid neutralization, fluegas desulfurization, sludge stabilization or other related industrial alkali applicationsQuicklime Graymont2024年9月10日 Hydrated lime is quicklime with water added, so its chemical name is calcium hydroxide However, hydrated lime is usually a fine, dry powder The water stays inside the calcium oxide particles—just like your body’s cells contain water but are solid rather than liquidLime vs Limestone Rock: Types and Uses of Each

Difference between quick lime, slaked lime, lime water and soda lime
2023年11月9日 What is slaked lime, quick lime, lime water What are their uses? Calcium hydroxide (also called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2 It is a colorless crystal or white powder and is obtained when calcium oxide is mixed with water It is used in sewage treatment,food industry and is also used as a insect Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kilnThis is accomplished by heating the material to above 825 °C (1,517 °F), a process called calcination or limeburning, to liberate a molecule of carbon dioxide (CO2), leaving quicklimeCalcium Oxide LimecoThe production of high calcium quicklime begins with the selective mining of chemically suitable calcium carbonate typically containing less than 5% magnesium carbonateHigh Calcium Quicklime GraymontHigh calcium quicklime (CaO) is produced when limestone, or calcium carbonate (CaCO 3), is heated in a kiln through the process of calcination CaCo 3 + heat > Cao = CO 2 After limestone with high calcium content is sourced from our quarries and underground mines, it is transported and processed through a series of crushers to reach a desired sizeHigh Calcium Quicklime Carmeuse

What Is Lime? Lime Association
2024年2月1日 During that process, carbon dioxide is driven off, leaving calcium oxide (lime) behind Society has made use of lime for centuries Here are just a few examples: In Ancient Rome, lime was used in construction for making mortar and concrete Romans mixed lime with volcanic ash to create a durable building material known as pozzolanic mortarCalcined Kaolin Powder is a kind of nonmetallic Calcium hydroxide Calcium oxide/quick lime White stone Limestone Activated carbon/Cylindrical activated carbon Heavy and Light Calcium Carbonate Gardening Color Ceramsite Planting ceramsite Medical stone Pumice Nutritional soil Coral sand Used for making daily ceramics, refractory Washed Kaolin/Calcined Kaolin Powder Hebei Yayang Types of Quick Lime Quick lime can be categorized based on its form and purity: 1 HighCalcium Lime: This type contains more than 95% calcium oxide It is produced from pure limestone and is used in processes where high purity is essential, such as in Understanding Quick Lime: Types, Properties, and Applications2023年10月27日 Quicklime is an alkaline substance produced from heating limestone in specialized kilns Humans have used quicklime since antiquity for construction, agriculture and metalwork applications It is still used in all of Quicklime: A Primer
.jpg)
Quick Lime Dry Calcium Oxide Manufacturer from
Manufacturer of Quick Lime Dry Calcium Oxide, Burnt Lime Stone, Uthaya 50kg CaO and White Washing Lime offered by Uthaya Chemicals, Chennai, Tamil Nadu Send 89% Response Rate Home: About Hydrated lime is also known as calcium hydroxide or slacked lime with CaOH2 chemical formula It is produced by mixing water to process Quick Lime and extract extra contaminants from Quick Lime to give the lime its purest form Hydrated Lime Powder Manufacturer Quick LimeCalcium oxide is a key ingredient for the process of making cement Calcium oxide is used to regenerate sodium hydroxide from sodium carbonate in the chemical recovery at Kraft pulp mills Technical Specifications of Calcium Oxide Powder : / Quick Lime Molecular Weight : 5608; Density : 33 g/ccm; Form : White Powder;Calcium Oxide Powder / Quick Lime Leading Supplier of Quicklime When a calcium limestone or chalk rock, that comprises mainly of calcium carbonate (CaCO 3), is heated in a kiln, it changes by a process called calcination into quicklime also known as 'burnt lime' and chemically is mainly calcium oxide (CaO), and the calcination process releases a gas from the rock which is carbon dioxide (CO 2) Hydrated LimeMaking Lime
.jpg)
10 Uses of Calcium Oxide in Daily Life Nanografi Nano Technology
The main ingredient is used in the process of making cement, and is calcium oxide As a broadly available and cheap alkali Total quicklime production of almost 50% is changed to calcium hydroxide before usage Drinking water is treated by using both hydrated and quicklime Petroleum industry2024年11月13日 CaO + H 2 O → Ca(OH) 2 This reaction is very exothermic and results in the creation of large clouds of steam Preparation of Calcium Oxide (quick lime) Calcium oxide can be made in a lime kiln by thermal breakdown of calcium carbonate (CaCO 3; mineral calcite)containing materials like limestone or seashells Calcination is the term for the process of Calcium Oxide Overview, Structure, Formula, Examples, Uses, FAQs2024年6月12日 When you pour water over highquality quicklime or milder garden lime, the calcium oxide in it converts to calcium hydroxide High temperatures are generated in the process This process is also known as burnt white lime Perhaps you have already heard of it However, if you only take calcium oxide, you will find that this effect is not long Make Heat Packs For Cooking Yourself With Quicklime Survival Newcrest Lime Ltd is a leading manufacturer and supplier of highquality lime products, including Quicklime, Hydrated Lime, Quicklime Powder, and Limestone, in Zambia We offer superiorquality lime products for different industrial, commercial, and agricultural purposesNewcrest Lime Limited LIME MANUFACTURER IN ZAMBIA