
What are the characteristics of calcite calcium carbonate crushing station

Calcite an overview ScienceDirect Topics
Calcite is the most common polymorph of calcium carbonate found in the Earth's crust, and it is the main component of limestone It plays a crucial role in geochemical systems by exchanging carbonate ions with aqueous solutions, influencing the chemical behavior of soils and ground calcium carbonate (GCC) produced by crushing limestone physically and PCC chemically formed through the calcination, hydration and carbonation of limestoneFormation Characteristics of Precipitated Calcium Carbonate by 2022年1月1日 The comparison of calcium oxide contents of different calcite samples showed that the calciteAF and the calciteBU were characterized by higher CaO contents, but silica The effects on the grinding parameters of chemical, morphological 2022年1月1日 The mechanism of precipitation of CaCO 3 has been widely studied, and it is divided into three main stages: i) predominant presence of Amorphous Calcium Carbonate Factors controlling and influencing polymorphism, morphology

Maximising the benefits of calcium carbonate in sustainable
2024年1月19日 In this context, we contend that due to the distinct characteristics of various calcium carbonate forms, there exists the substantial potential to maximise their technical, 2018年1月1日 The carbonate minerals are especially important minerals found in the ocean as the skeletons and tests of marine organisms and as cements in marine sediments Calcite Carbonate Minerals and the CO 2 Carbonic Acid System Springer2020年11月23日 During incorporation into carbonate crystal lattices, metal ions could eventually be adsorbed on the solid surface Taking into account the rapid deposition of calcite from Coprecipitation of metal ions into calcite: an estimation of 2010年12月31日 With the contribution of submicroscopic techniques (eg, SEM), progress has been made in the understanding of the relation between the biological activity and the (PDF) Calcium Carbonate Features ResearchGate
.jpg)
Features and characteristics of calcium carbonate
Calcium carbonate is composed of three elements which are of particular importance for all organic and inorganic material on our planet: carbon, oxygen and calcium Download to read 2024年7月11日 Structure of Calcium Carbonate Calcium carbonate is characterized by its unique chemical structure, represented by the formula CaCO₃At its core, this compound consists of one calcium (Ca) ion bonded to Calcium Carbonate(CaCo₃) Definition, Structure, Limestone is a sedimentary rock that is composed primarily of calcite It forms from both the chemical precipitation of calcium carbonate and the transformation of shell, coral, fecal and algal debris into calcite during diagenesis Limestone Calcite Mineral Uses and Properties Geology2016年12月22日 From the PDF data, it is not likely that ACC is a nanocrystalline “version” of crystalline calcium carbonate However, other studies have pointed some similarities between ACC and some of the CaCO 3 polymorphs For Structural Characteristics and the Occurrence of
.jpg)
Carbonate Rocks Geology is the Way
Origin of carbonate sediments Calcium carbonate occurs dissolved in seawater and fresh waters Calcium derives from the weathering of Cabearing minerals in rocks, like plagioclase, and it is present in water as Ca 2+ ions Atmospheric CO 2 dissolves in water producing H 2 CO 3 (carbonic acid), a weak acid, following the reaction: CO 2 (gas) + H 2 O(liquid) ⇌ H 2 CO 3Crystal structure of calcite Calcium carbonate shares the typical properties of other carbonatesNotably it reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water:; CaCO 3 (s) + 2 H + (aq) → Ca 2+ (aq) + CO 2 (g) + H 2 O(l) releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above Calcium carbonate Wikiwand2024年1月17日 Calcite crystals showing rhombohedral cleavage Credit: Siim Sepp Calcite is a carbonate mineral that forms a significant part of the Earth’s crustIt’s one of the most common types of Calcite geology: mineral properties, crystal structure, uses ZME Key wordsprecipitated calcium carbonate, carbonation process, calcite, aragonite, adipic acid 1 Introduction Calcium carbonate is the main component of limestone, which is the most abundant among nonmetallic minerals, and there are three anhydrous crystalline polymorphs such as calcite, aragonite, and vaterite1,2) The crystalFormation Characteristics of Precipitated Calcium Carbonate by
.jpg)
Characteristics of the Treated Ground Calcium Carbonate Powder
2009年2月1日 Calcium carbonate can be treated in two ways: granulated calcium carbonate or GCC (Ground Calcium Carbonate) made by grinding natural calcite [1] and precipitated calcium carbonate (PCC) with 2021年7月8日 Meals: Different types of calcium vary in whether they're absorbed best with or without foodCalcium carbonate should be taken with meals Calcium citrate should be taken on an empty stomach Medications: Calcium should not be taken with certain medications, including antibiotics, iron supplements, high blood pressure medications, and othersCalcium Citrate vs Calcium Carbonate: Which to Take? Verywell Calcium carbonate is one of the most useful and versatile materials known to man This family of essential minerals comprises more than four percent of the earth’s crust and is found worldwide It is produced by the sedimentation of Calcium Carbonate Essential Minerals Association2023年12月7日 The equipment used in the manufacturing process for calcium carbonate varies depending on the type of calcium carbonate being produced For natural calcium carbonate, the equipment typically includes crusher, What is the steps in the Calcium Carbonate

Marble Properties, Uses, Formation Geology Science
2023年8月21日 Major minerals of Marble: Calcite; Accessory minerals of Marble: Diopside, tremolite, actinolite, dolomite; transparent emerald, the green variety of beryl on calcite (marble) matrix Origin of Marble Marble is a type of Calcium Carbonate Formula It is a chemical compound with the chemical formula CaCO 3; It is a white insoluble powderlike substance which occurs naturally in minerals, chalk, marble, limestone, calcite, shells, pearl, etc; Medicinally, it is used as an antacid or as a Limestone: Calcium Carbonate (CaCO3) Uses, Preparation, 2024年10月26日 Calcium carbonate (CaCO3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals Calcium carbonate is either a white powder or a colorless crystal When heated, itCalcium carbonate Formula, Uses, Names, Facts Britannica2024年8月3日 Microbialinduced calcium carbonate precipitation (MICP) can enhance the physical properties of recycled aggregates Compared to traditional technologies, MICP offers environmental benefits and produces no pollution However, its mineralization efficacy is significantly influenced by the process parameters To investigate this, an MICP mineralization Factors Affecting the Physical Properties of Microbial Induced Calcium

Soil bacteria that precipitate calcium carbonate: Mechanism and
2017年1月1日 Bacteria with ureasic activity are microorganisms found in soil that in presence of urea and calcium, they can produce calcium carbonate, a process known as microbiologically induced calcium Download scientific diagram FTIR spectra of calcium carbonate precipitates at different time intervals (A) One day, (B) four days [Ca 2+ ]/[H 4 dhpta] ¼ 4 : 1 The absorption bands at 865 cm FTIR spectra of calcium carbonate precipitates at different 2024年3月21日 Calcium carbonate is an inorganic followed by carbon dioxide 4397% Due to the characteristics of calcium carbonate, it exists widely in nature and is used in various industries drying and pulverization Heavy calcium carbonate is physical grinding and crushing of marble, limestone, dolomite and calcite through General Introduction of Calcium Carbonate Powder ChemicalBookDownload scientific diagram Three polymorphs of calcium carbonate; Calcite, Aragonite and Vaterite from publication: Calcium carbonate nanoparticles; Potential in bone and tooth disorders Three polymorphs of calcium carbonate; Calcite, Aragonite and
.jpg)
Calcite Crystal Vs Other Carbonate Minerals: A
Calcite Crystal and other carbonate minerals are interesting geological formations that have fascinated scientists and enthusiasts They have unique properties and structures, making them a fascinating subject of study In this article, we will Keywords: Calcium carbonate, Stearic acid, Treatment method, Void content Abstract Calcium carbonate (CaCO 3) is often treated with stearic acid (SA) to decrease its polarity However, the method of application of the SA treatments has a strong influence on Characteristics of the treated calcium carbonate particles with 2023年6月25日 Crushing: The calcium carbonate stones just mined from the quarry are relatively large, and they need to be crushed by a jaw crusher and a hammer crusher in turn to the feed fineness (10mm20mm) that can enter the Guide to Calcium Carbonate Grinding: Mills, Tips, and Solubility and Body Fluids Erich Königsberger, LanChi Königsberger, in Thermodynamics, Solubility and Environmental Issues, 2007 335 Pancreatic Stones Calcium carbonate is a major constituent of pancreatic stones (consisting of ca 95% calcite) and is found occasionally in salivary stones and many pigment gallstones, since these three gastrointestinal secretions Calcium Carbonate an overview ScienceDirect Topics

(PDF) Effect of Calcium Carbonate Replacement on Workability and
2015年7月1日 The surface analysis of polymorphic calcium carbonate (CaCO3) compounds, namely, calcite, aragonite, and vaterite were carried out by Xray photoelectron spectroscopy (XPS)2024年1月19日 With the recognised reactive role of calcium carbonate in PC, there is a growing interest in harnessing various forms of calcium carbonate to enhance the performance of different cement typesMaximising the benefits of calcium carbonate in sustainable The number one similarity between Calcite and Aragonite is that they are both mineral features of Calcium Carbonate Calcite and Aragonite have the exact chemical formula, which is CACO3 They are the two most prevalent phases of Calcium Carbonate Either Calcite or Aragonite creates the husks and skeletons of numerous marine lifeCalcite vs Aragonite: What Are They, And What’s The Difference?2010年12月31日 PDF The precipitation of calcium carbonate is common in soils and regoliths, Calciterich groundmass with calcitic crystallitic bfabric in calcareous soil (southern Tunisia)(PDF) Calcium Carbonate Features ResearchGate
.jpg)
Characteristics of the Treated Ground Calcium Carbonate
Keywords: ground calcium carbonate, stearic acid, flowability, floodability, dispersive component of surface free energy (D S), inverse gas chromatography (IGC) 1 Introduction Ground calcium carbonate (GCC) is the most widely used filler in the plastics, rubber, paper, paint and ink industry1) It is usually obtained by grinding natural 2018年8月11日 Calcite is calcium carbonate (CaCO 3) and belongs to trigonal (rhombohedral) crystal system It occurs in a wide variety of crystal habits rhombohedrons, scalenohedrons, tabular and prismatic crystals and just about everything in Calcite Mineral Properties, Photos and Occurence2007年4月1日 Request PDF The Role of Calcium Carbonate in Cement Hydration Limestone, mainly consisting of calcite, is a permitted additive to Portland cements often up to a 5 wt% limit It is shown by The Role of Calcium Carbonate in Cement Hydration2016年12月1日 The synthesis of a large area calcium carbonate (CaCO3) film was carried out under room temperature and atmospheric pressure The crystallographic, thermal, and mechanical properties of amorphous Behavior and characteristics of amorphous calcium carbonate and calcite

Carbonate Minerals and the CO2Carbonic Acid System
2018年1月1日 Cacarbonates are primarily inorganic and biogenic products of the marine environment CaCO 3 is a first precipitate in an evaporative sequence of seawater, and later precipitates are Na and K chlorides and sulfates In continental waters, however, the anions HCO 3 − and CO 3 2− are usually more abundant than Cl − and SO 4 2−, and the minerals 2021年11月15日 Carbonate minerals are constituted by the combination of one (CO 3) 2− ion and various monovalent or divalent cations Rhombohedral carbonates such as calcite, magnesite, siderite, and rhodochrosite crystallize in the R3c group [14])Dolomite and ankerite minerals are structurally similar to the rhombohedral carbonates (R3 group) but with different Identification and composition of carbonate minerals of the calcite Keywords: ground calcium carbonate, stearic acid, flowability, floodability, dispersive component of surface free energy (D S), inverse gas chromatography (IGC) 1 Introduction Ground calcium carbonate (GCC) is the most widely used filler in the plastics, rubber, paper, paint and ink industry1) It is usually obtained by grinding natural Characteristics of the Treated Ground Calcium Carbonate Powder hand, consist of calcium carbonate (CaC0 3) 1 Features and characteristics of calcium carbonate Calcium carbonate is composed of three ele ments which are of particular importance for all organic and inorganic material on our planet: carbon, oxygen and calcium The elements carbon, oxygen and calcium have their origin in the interior of 1 Features and characteristics of calcium carbonate Springer
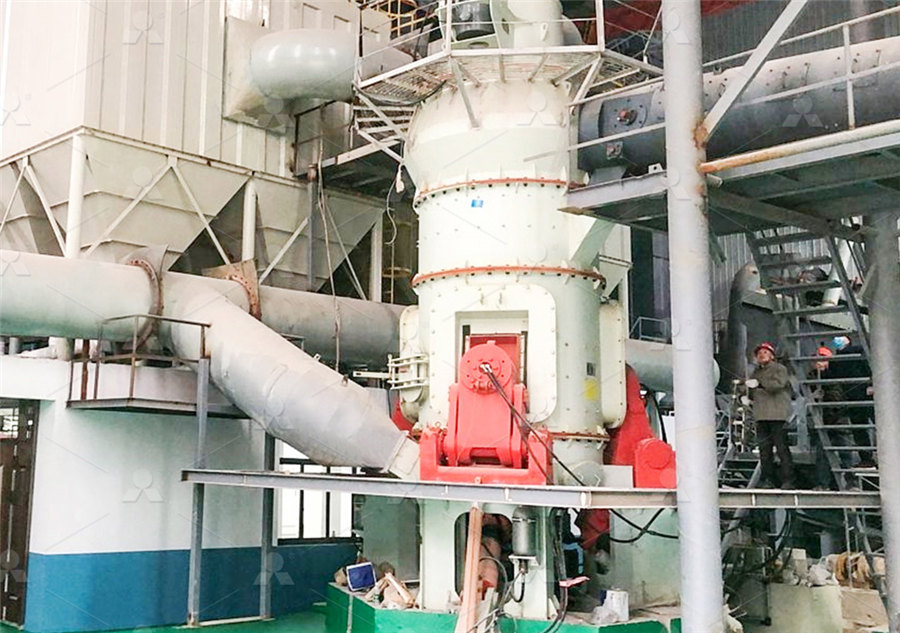
Calcium Carbonate Manufacturing Process and Equipment
2021年12月20日 The Importance of Calcium Carbonate Calcium carbonate (CaCO3) comprises more than 4% of the earth’s crust and is found worldwide Its most common natural forms are chalk, limestone, and marble (produced by the sedimentation of small fossilized shellfish, snails, and coral over millions of years)2023年10月21日 Limestone is a sedimentary rock primarily composed of calcium carbonate (CaCO3) in the form of mineral calcite or aragoniteIt is one of the most common and widely distributed rocks on Earth, with a wide range of uses in various industries and natural settings Limestone forms through the accumulation and compaction of marine organisms, primarily the Limestone Types, Properties, Composition, Formation, Uses2023年9月12日 Calcite dissolves easily in weak acids including vinegar and gives off bubbles of CO 2 A very special characteristic of transparent calcite is birefringence (double refraction) A line viewed through the crystal will appear double One characteristic that makes calcite so interesting to collectors is the variety of crystal habitsWhat is Calcite Natural History Museum of UtahCalcium carbonate is a kind of inorganic compounds, white or colorless crystalline powder, odorless, tasteless, commonly known as grey stone, limestone, stone powder, marble and calcite it is a kind of compound, alkaline, basically does not dissolve in water, soluble in acid, found in aragonite and calcite, chalk, limestone, marble, travertine and so on within the rock, it is 4 Steps to Build a Calcium Carbonate Processing Plant Zenith













