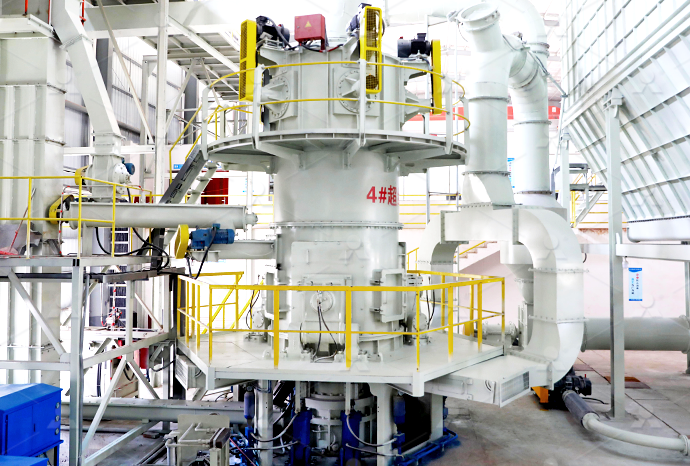
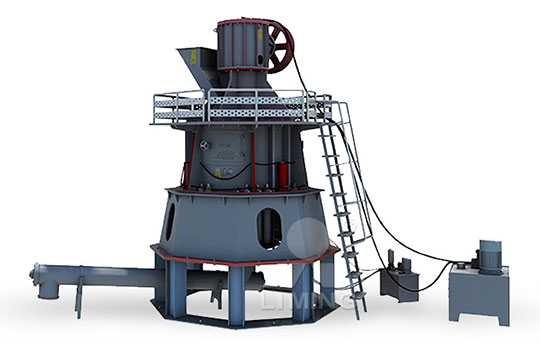
How many times to roll the surface quicklime and calcium carbonate of Raymond mill
.jpg)
PaperThe effects of limestone characteristics and calcination
2001年4月1日 Samples of limestones were calcined at four different temperatures (900°C, 1000°C, 1100°C, 1200°C) for 24 h for the production of quicklime The quicklime was hydrated, with the addition of appropriate amount of water until slaked lime putty with adequate 2012年1月1日 This article presents the results of an experiment conducted to determine how length of calcination of selected limestones affects the slaking characteristics of quicklime, The Effect of Calcination Time upon the Slaking Properties of Calcium oxide is traditionally known as quicklime If you add water to calcium oxide, you get calcium hydroxide (slaked lime) CaO(s) + H 2 O(l) Ca(OH) 2 (s) There is a useful bit of video limestone, quicklime and slaked lime chemguide2001年4月1日 Test results indicate that the lower the limestone calcination temperature, the more reactive the produced quicklime The optimum calcination temperature is ∼900°C, which (PDF) The Effects of Limestone Characteristics and Calcination

Lime An Introduction
Quicklime is produced by heating any material containing calcium carbonate to a temperature of around 1000°C for several hours In this process, known as 'calcining' or simply 'burning', the The quicklime is not stable and, when cooled, will spontaneously react with CO 2 from the air until, after enough time, it will be completely converted back to calcium carbonate unless Calcium oxide Wikipedia2021年3月3日 The recarbonation of quicklime takes place at room temperatures via surface hydration of the quicklime by atmospheric water vapor and carbonation of calcium hydroxide to Lime SpringerLinkQuicklime is a calcium oxide formed to release carbon dioxide by calcinating calcium carbonate (limestone) Quicklime is also referred to as handpicked lime, burnt lime, lump lime, calcining Quicklime Preparation, Properties, and Applications with FAQs

The Versatile Chemical Lime
Quicklime, the product of calcination of limestone, consists of the oxides of calcium and magnesium The primary forms of quicklime are: High calcium quicklime derived from 2024年11月8日 Quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate Quicklime Formula, Uses, Definition Britannica2023年6月25日 Crushing: The calcium carbonate stones just mined from the quarry are relatively large, and they need to be crushed by a jaw crusher and a hammer crusher in turn to the feed fineness (10mm20mm) that can enter the Guide to Calcium Carbonate Grinding: Mills, Tips, and When heated above $\sim 900^{\circ} \mathrm{C}$, limestone (calcium carbonate) decomposes to quicklime (calcium oxide), which is used to make cement, 01:13 If $1011 \mathrm{~g}$ of limestone decomposes by heat to give $851 \mathrm{~g}$ of solid calcium oxide and carbon dioxide gas, what is the mass of Limestone (CaCO 3 ) is decomposed by heating to quicklime

What is Calcium Carbonate? (with pictures)
2024年5月21日 Calcium carbonate is an important chemical compound made up of one atom of calcium bonded to one atom of carbon and three atoms of oxygenIts molecular formula is CaCO mon names for this compound Slaking residence time is a factor to consider when using a continuous slaker (detention, ball mill and paste) As quicklime constantly moves through these types of slaker, it is important to ensure the quicklime spends enough time in the slaker to complete the slaking reaction Typically, North American quicklimesLIME SLAKING 101 Carmeuse2021年9月2日 In the lime slurry formed after quicklime slaked, the lime particles form a calcium hydroxide colloidal structure, the particles are extremely fine (particle size is about 1μm), the specific surface area is large (up to 1030m2/g), and a thicker layer is adsorbed on the surface Water film can absorb a large amount of water, so it has a strong ability to retain water, that is, The difference between quicklime and hydrated limeBe sure to answer all parts Limestone (CaCO3) is decomposed by heating to quicklime (CaO) and carbon dioxide Calculate how many grams of quicklime can be produced from 40 kg of limestone × 10 g Enter your answer in scientific notationSOLVED: Be sure to answer all parts Limestone (CaCO3) is

Calcium Oxide (CaO) Preparation, Properties Uses of Quicklime
Calcium Oxide, also known as quicklime, lime water, or burnt lime, is a chemical compound with the formula CaO Learn about the preparation, properties, and uses of Calcium oxide hereThe quality of quicklime is affected by several factors related to the limestone feed and the calcination process These include the chemical composition and crystalline structure of the limestone, as well as operating conditions in the kiln such as temperature, particle size of the limestone, rate of temperature rise, retention time, and CO2 concentration Controlling these Factors Affecting The Quality of Quicklime PDF Sodium Carbonate 2008年1月2日 Quicklime is not only an important raw material for the steel and nanocalcium carbonate industries but also a key carrier for capturing carbon dioxide in the fight against global warming, and its Factors influencing the reactivity of quicklime ResearchGate2024年10月26日 Calcium carbonate is either a white powder or a colorless crystal When heated, it produces carbon dioxide and calcium oxide (also called quicklime) Calcium carbonate has a molecular weight of 1001 grams per mole Calcium carbonate occurs naturally in three mineral forms: calcite, aragonite, and vateriteCalcium carbonate Formula, Uses, Names, Facts Britannica

(PDF) Natural and enhanced carbonation of lime in
2021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination2016年10月19日 Quicklime (CaO) is obtained by calcination of limestones containing calcium carbonate at 900 1000 o C (Anıl and Kılıç, 2006) Slaked lime is calcium hydroxide (Ca(OH) 2 ) which isOptimization of Grinding Parameters of Quicklime CaO at2014年5月1日 In addition, hydrated lime (calcium hydroxide) can be used to create a sterile ''crust'' on the surface of remains or carcasses because, when exposed to air it absorbs carbon dioxide and releases Longterm effects of hydrated lime and quicklime on the decay of 2001年4月1日 Table 2 reports the results of chemical analyses performed by calcimetry, DTA/TG, and AAS The weight loss above 600°C, measured by TG, is attributed to the CO 2 from the decomposition of calcium carbonate [8]Comparing the percentage values of CO 2 measured by calcimetry and DTA/TG, it is evident that the values are very high and similar to each otherThe effects of limestone characteristics and calcination temperature to
.jpg)
Understanding Lime: an introduction to forms of lime and
2013年3月3日 One type contains a lot of Magnesium in a similar form and we call that Dolomite lime Dolomite's uses are similar to regular limestone The limestone form, including shells of all kinds, is Calcium Carbonate It is calcium with 3 carbon atoms attached to it You know, carbon as in carbon dioxide the famous greenhouse gasCalcium Carbonate + Heat → Calcium Oxide (quicklime) + Carbon Dioxide CaCO 3 + Heat → CaO + CO 2 Processing The calcium oxide produced is in the form of white lumps It can either be crushed or ground, after which, it can be mixed with specific impurities to form compositesWhat is Quicklime and How is it Made? Science Struck2024年11月8日 quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxideAt room temperature, CaO will spontaneously absorb carbon dioxide from the atmosphere, reversing the reactionIt will also absorb water, converting itself into Quicklime Formula, Uses, Definition Britannica1984年1月1日 The spontaneous formation of calcium carbonate (CaCO 3 ) scale, also known as limescale, is an ordinary phenomenon long known to affect the efficiency of water supply networks, boilers and heat Precipitation of Calcium Carbonate in Aqueous Solutions
.jpg)
How to Make Quicklime: 10 Steps (with Pictures) wikiHow
2024年4月16日 Because whatever rock you’ll be using as a material to make quicklime will not contain 100% calcium carbonate, you’ll need to acquire more rock than you’ll need for the amount of quicklime you want to make You will need 18 units of raw calcium carbonate for every 1 unit of quicklime you want to makeCalcium Carbonate (CaCO3) Calcium carbonate molecular formula is CaCO3 Visit BYJU'S to understand the properties, structure and Uses of calcium carbonate (CaCO3) explained by India's best teachers It can also be made chemically by combining quicklime (calcium oxide, CaO) with water to produce calcium hydroxide (Ca(OH) Calcium Carbonate (CaCO3) Structure, Properties, Uses of Calcium 2010年1月1日 It is commonly used as sand in the production of various types of concrete including foam concrete [17] with a chemical composition that is dominated by calcium carbonate which can be transformed The effects of limestone characteristic, granulation and calcination Calcium carbonate nanocomposites Y Lin, CM Chan, in Advances in Polymer Nanocomposites, 2012 31 Introduction: applications of calcium carbonate nanoparticles Calcium carbonate particles have been used in the plastics industry for many years The original purpose of adding ground calcium carbonate (GCC) particles as filler material for plastics was to Calcium Carbonate an overview ScienceDirect Topics

Impact of calcination temperature and time on quicklime slaking
Quicklime is an industrial bulk product, and is used in many industrial applications, eg steel production, or paper and pulp industry [1][2], the slaking reactivity is one of the main parameters in determining the quicklimes quality [3] Quicklime is produced in industrial kilns, where limestone is heated to calcination temperatures by theA large range of fillers and coating pigments for best whiteness, printability and costeffective fiber substitution We offer a wide range of carbonate products that differ in particle size, particle size distribution and brightness to suit all paper and board application needs Ground Calcium Carbonate (GCC) is composed of the crystalline mineral calcite, which occurs naturally in the Calcium carbonate for paper and board Imerysthan quicklime Quicklime and hydrated lime have a very wide and well documented variety of uses This conversion of calcium carbonate to calcium oxide is achieved by heating the limestone to a temperature high enough (eg 1000 C in a lime kiln) to 'drive off' carbon dioxide, CO 2 The equation for this process, with the approximateHOW TO CALCULATE EFFICIENCY OF YOUR LIME BURNING Anhydrous calcium sulfate may also be used provided it has been treated with a colorchanging indicator to show when the desiccant has lost its effectiveness Calcium chloride and silica gel are not satisfactory desiccants for this type of analysis Standard Test Methods for Chemical Analysis of Limestone, Quicklime

Effects of hydrated lime and quicklime on the decay of buried
2012年1月1日 Raman spectroscopy at 785 nm and 1064 nm provided information about the impact of environmental conditions on the extent of deterioration to these samples, the presence of organics and the the surface impurities reacted with calcium forming Larnite, Gehlenite, Åkermanite and Merwinite, reducing the quicklime quality The results showed that the limestone surface layer comprised 12Characterization of Limestone Surface Impurities and Resulting Where a farmer talks about "liming" a field, they are most likely to be using calcium carbonate Quicklime, CaO, and slaked lime, Ca(OH) 2 When calcium carbonate is heated strongly, it decomposes to give calcium oxide and carbon dioxide CaCO 3 (s) CaO(s) + CO 2 (g)limestone, quicklime and slaked lime chemguideQuicklime is an industrial bulk product, and is used in many industrial applications, eg steel production, or paper and pulp industry [1][2], the slaking reactivity is one of the main parameters in determining the quicklimes quality [3] Quicklime is produced in industrial kilns, where limestone is heated to calcination temperatures by theImpact of calcination temperature and time on quicklime slaking

Impact of Limestone Surface Impurities on Quicklime
2024年2月27日 The results suggest that impurities found on the surface of the lime kiln limestone feed reduce the main quality parameter of the quicklime products, ie, calcium oxide, CaO (s), content by 08 2022年10月12日 According to current averages, soil in the embankment has been further dehydrated over the past 23 years at a rate of 138% (w/w) As a result, quicklimetreated soil had an average calcium carbonate content of 35 mg/w (w/w)The Many Uses Of Quicklime – AccessibleGardensCalcium carbonate decomposes at high temperatures to form carbon dioxide and calcium oxide a Write the balanced chemical equation for this reaction b How many grams of calcium carbonate will you need to form 345 liters of carbon dioxide? Limestone (CaCO3) is used to remove acidic pollutants from smokestack flue gasesLimestone CaCO3 is decomposed by heating to quicklime CaO Calculate how many grams of quickline can be produced from 10kg of limestone limestone is decomposed by heating to quicklime and carbon dioxide Here’s the best way to solve itSolved limestone is decomposed by heating to quicklime and
.jpg)
QUICK LIME AND BYPRODUCTS PEC Consulting Group
Crude sugar juice (sucrose) is treated with lime to raise the pH and to precipitate calcium salts of organic and inorganic acids These insoluble compounds are filtered Carbon dioxide is then passed through the suspension to precipitate excess lime as calcium carbonate Precipitated carbonate sludge is filtered out1 Calcium Carbonate [CaCO3] is the chemical description for pure or highcalcium lime products, normally found in nature (limestone, oyster shells) This material is sometimes sold crushed for use in lawn care and agricultural it is not suitable for mortar 2 Calcium Oxide [CaO] or Quicklime is produced by firing Calcium Carbonate to 900° and driving off CO2Lime types and their meanings explained in our Glossary!2020年10月16日 Let the mass of quicklime formed is “x” CaCo 3 → CaO + CO 2 100g x 44g According to the law of conservation of mass, the sum of the masses of reactants is equal to the sum of the masses of products formedIf 100 g of calcium carbonate on heating produces 44g of Methods Equipment Finished product(D97/ μ m) Features Dry grinding process Raymond roller mill 25150 1Applicable materials: medium and low hardness; 2Product features: medium and lowend products, suitable for the production of coarse powder;How to Choose a Calcium Carbonate Grinding Mill DASWELL
.jpg)
Calcium Carbonate in the Concrete Industry Noah Chemicals
2022年3月8日 What is Calcium Carbonate (CaCO3) and how it is used in the Concrete Industry? The higher the slump rate, the higher the concrete’s workability, facilitating a long enough work time so that it can level itself and be more easily pushed, 2020年3月20日 Properties Chemical Calcium oxide can be reacted, or slaked, with water, releasing large amounts of heat and producing calcium hydroxideIn open air, it reacts with water vapor and carbon dioxide, slowly converting to calcium carbonate over time It is used as a component of many cement mixes, and can be reacted with acids to form calcium saltsCalcium oxide Sciencemadness Wiki